Difference between revisions of "Quell relief"
(→Public Policy: update) |
(→Company & People: update) |
||
| Line 74: | Line 74: | ||
=== Company & People === | === Company & People === | ||
<!-- This is a list of important people behind the technology. Note: It is not necessary to list absolutely everyone. --> | <!-- This is a list of important people behind the technology. Note: It is not necessary to list absolutely everyone. --> | ||
| + | |||
| + | The company was found as a spin-off from has a seat in NEUROMETRIX, INC. 1000 Winter St,Waltham , Massachusetts 02451. and was founded as a spinoff of the Harvard-MIT partition of Health Sciences and Technology. | ||
== Important Dates == | == Important Dates == | ||
Revision as of 03:03, 10 November 2015
Quell wearable is device designated for treatment of chronic pain. It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief.
| QUELL RELIEF | |
|---|---|

|
|
| Category | Therapeutic wearables |
| Developer | NeuroMetrix |
| Announced | Oct 2015 |
| Released | Consumers: 2015 |
| Price | 249 USD |
| Weight | 62 g |
| Dimensions | 98 x 74 x 11 mm |
| Controls | smartphone |
| Standalone[1] | |
| https://www.quellrelief.com/product | |
Contents
Main characteristics
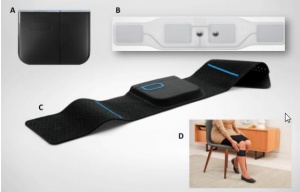
Purpose
Company & People
The company was found as a spin-off from has a seat in NEUROMETRIX, INC. 1000 Winter St,Waltham , Massachusetts 02451. and was founded as a spinoff of the Harvard-MIT partition of Health Sciences and Technology.
Important Dates
1965 - Melzack and Wall proposed first conceptual model for mechanism which could lead to pain relief
1970s - Developement of TENS (Transcutaneous electrical nerve stimulation)
Ethical Issues
Health Risks
Official statement about side effects is that there are none[1]. Usage is 100% drug free, with clearance by FDA (Food and Drug Administration)
Enhancement/Therapy/Treatment
Quell wearable device has primarily therapeutical function. It was developed on purpose to palliate chronical pain which basically has origin in these four diseases:
Public & Media Impact and Presentation
Public Policy
Food and Drug Administration contains database consisting of three chapters (I. Food and Drug Administration, Department of Healt and Human Services; II. Drug Enforcement Administration, Department of Justice; III. Office of National Drug Control Policy), where each device is sorted and classified according to specific properties. This Database comes actually under the general database called Electronic Code of Federal Regulations (eCFR). It the chapter I, Quell describtion consists of a) identification and b) classification. Quell is kept under regulation number: 882.5890, and it is identified as "A transcutaneous electrical nerve stimulator for pain relief is a device used to apply an electrical current to electrodes on a patient's skin to treat pain"[3] . Further, it is classified with mark "Class II (performance standards)"[4]. The latter category features most of medical devices (FDA stated 43%), including for example wheelchairs of pregnancy test kits[5].
Related Technologies, Project or Scientific Research
References
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ Shai N. Gozani, Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf
- ↑ Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890
- ↑ Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890
- ↑ Medical device classification list on: http://www.fda.gov/MedicalDevices/ResourcesforYou/Consumers/ucm142523.htm