Difference between revisions of "Quell relief"
(→Purpose: update) |
(→Main characteristics: update) |
||
| Line 66: | Line 66: | ||
== Main characteristics == | == Main characteristics == | ||
<!-- This section should describe the technology in more detail. Here should be information about the used hardware and software, available features, chemical composition and so on, provided that they are available. Second half of this section should offer information on history of the technology. When it was created, unveiled, developed, announced to the public or when it was available to purchase. Anything related to the technology that can be pinpointed to a certain date should be in this section together with relevant commentary.--> | <!-- This section should describe the technology in more detail. Here should be information about the used hardware and software, available features, chemical composition and so on, provided that they are available. Second half of this section should offer information on history of the technology. When it was created, unveiled, developed, announced to the public or when it was available to purchase. Anything related to the technology that can be pinpointed to a certain date should be in this section together with relevant commentary.--> | ||
| + | |||
| + | |||
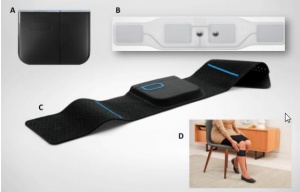
[[File:Quell-components.jpg|thumbnail|A) A therapy pod, B) An electrode array, C) A Band, D) An example of usage on upper calf, place of effect<ref>Shai N. Gozani, Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf</ref>]] | [[File:Quell-components.jpg|thumbnail|A) A therapy pod, B) An electrode array, C) A Band, D) An example of usage on upper calf, place of effect<ref>Shai N. Gozani, Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf</ref>]] | ||
| Line 72: | Line 74: | ||
<!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | <!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | ||
| − | Quell wearable is device designated for treatment of chronic pain. It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief. | + | Quell wearable is device designated for treatment of chronic pain, nerve deseases and disrupted sleep<ref>Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html</ref>. It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief. |
=== Company & People === | === Company & People === | ||
Revision as of 06:44, 10 November 2015
Quell wearable is device designated for treatment of chronic pain. It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief.
| QUELL RELIEF | |
|---|---|

|
|
| Category | Therapeutic wearables |
| Developer | NeuroMetrix |
| Announced | Oct 2015 |
| Released | Consumers: 2015 |
| Price | 249 USD |
| Weight | 62 g |
| Dimensions | 98 x 74 x 11 mm |
| Controls | smartphone |
| Standalone[1] | |
| https://www.quellrelief.com/product | |
Contents
Main characteristics
Purpose
Quell wearable is device designated for treatment of chronic pain, nerve deseases and disrupted sleep[3]. It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief.
Company & People
Neurometrix is company directed on developing health-care wearable technology with main effort on sleep disorders, chronical pain, and nerve deseases treatment. The company, having a seat in Waltham Massachusetts, was founded in 1996 as a spinoff of the Harvard-MIT partition of Health Sciences and Technology.[4][5]
Currently Neurometrix offers four products in total[6]:
Quell™ - Wearable device for paliative purpose
SENSUS® - Therapy device available on prescription. It is based on TENS technology also for pain relief without any drug usage.
DPNCheck® - Nerve conduction test for evaluating systemic neuropathies such as diabetic peripheral neuropathy (DPN), its detection and monitoring.
ADVANCE™ System
People in the lead of the company (the complete description is here):
Shai N. Gozani, M.D., Ph.D. - President, Chief Executive Officer and Director
Thomas T. Higgins - Senior Vice President and Chief Financial Officer
Frank McGillin - Senior Vice President and General Manager, Consumer
Michael J. MacDonald - Senior Vice President of Commercial Operations
Juan Kong, Ph.D. - Senior Vice President of Research and Intellectual Property
Neurometrix stated also contact persons for media, which is Laura Wagstaff[7], and Thomas T. Higgins as contact person for investor relations[8].
Important Dates
1965 - Melzack and Wall proposed first conceptual model for mechanism which could lead to pain relief
1970s - Developement of TENS (Transcutaneous electrical nerve stimulation)
Ethical Issues
Health Risks
Official statement about side effects is that there are none[1]. Usage is 100% drug free, with clearance by FDA (Food and Drug Administration) received in 2014.
Enhancement/Therapy/Treatment
Quell wearable device has primarily therapeutical function. It was developed on purpose to palliate chronical pain which basically has origin in these four diseases:
Public & Media Impact and Presentation
Advertising video found right on the Quell product website.
Public Policy
Quell received clearance from Food and Drug Administration, which is federal agency as part of Department of Health and Human Services. FDA is connected to the database on U.S. Government Publishing Office, Electronic Code of Federal Regulations (eCFR). This eCFR contains also of three chapters (I. Food and Drug Administration, Department of Healt and Human Services; II. Drug Enforcement Administration, Department of Justice; III. Office of National Drug Control Policy), where medical devices are sorted and classified according to specific properties, difficulties of usage, dangerousness, etc. It the chapter I, Quell description consists of a) identification and b) classification. Quell is kept under regulation number: 882.5890, and it is identified as "A transcutaneous electrical nerve stimulator for pain relief is a device used to apply an electrical current to electrodes on a patient's skin to treat pain"[9] . Further, it is classified with a "Class II (performance standards)"[10]. The latter category features most of medical devices (FDA stated 43%), including for example wheelchairs of pregnancy test kits[11].
Related Technologies, Project or Scientific Research
References
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ Shai N. Gozani, Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf
- ↑ Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html
- ↑ Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html
- ↑ NeuroMetrix Launches Quell™ Wearable Pain Relief Technology: http://www.businesswire.com/news/home/20150615005489/en/
- ↑ Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html
- ↑ NeuroMetrix Launches Quell™ Wearable Pain Relief Technology: http://www.businesswire.com/news/home/20150615005489/en/
- ↑ NeuroMetrix Launches Quell™ Wearable Pain Relief Technology: http://www.businesswire.com/news/home/20150615005489/en/
- ↑ Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890
- ↑ Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890
- ↑ Medical device classification list on: http://www.fda.gov/MedicalDevices/ResourcesforYou/Consumers/ucm142523.htm