Difference between revisions of "Quell relief"
(→Health Risks: update) |
(→TENS Research: update) |
||
| (56 intermediate revisions by 2 users not shown) | |||
| Line 22: | Line 22: | ||
<tr> | <tr> | ||
<th scope="row">Announced</th> | <th scope="row">Announced</th> | ||
| − | <td>[[Announced in:: | + | <td>[[Announced in::Mar 2015]]<ref>NeuroMetrix Launches Indiegogo Campaign for Quell™ Wearable Pain Relief Technology: http://investor.neurometrix.com/releasedetail.cfm?ReleaseID=899167, December 15, 2015</ref></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
<th scope="row">Released</th> | <th scope="row">Released</th> | ||
| − | <td>Consumers: [[Released for consumers::2015]]</td> | + | <td>Consumers: [[Released for consumers::May 2015]] <ref>NeuroMetrix Announces First Shipments of Quell™ Wearable Pain Relief Device: http://investor.neurometrix.com/releasedetail.cfm?releaseid=912945, December 15, 2015</ref></td> |
</tr> | </tr> | ||
| Line 34: | Line 34: | ||
<td>[[Costs::249]] USD</td> | <td>[[Costs::249]] USD</td> | ||
</tr> | </tr> | ||
| − | |||
<tr> | <tr> | ||
| Line 41: | Line 40: | ||
</tr> | </tr> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<tr> | <tr> | ||
<th scope="row">Controls</th> | <th scope="row">Controls</th> | ||
| Line 60: | Line 55: | ||
</table> | </table> | ||
| − | Quell is wearable device developed in purpose to provide palliative treatment for people suffering from chronical pain. Quell is commercially available, thus customers don't need any prescription from a doctor. In present, it is the only palliative wearable, which is approved by FDA to be used during sleep. Quell wearable is presented by two technological improvements, which is OptiTherapy™ electrode and transcutaneous electrical nerve stimulation (TENS), method allowing sending electrical pulses into afferent nerves through the skin. The technologie of TENS is not new, but it was developed on knowledge about possibility to heal with electricity, which was described already in 18th century<ref>Johnson, Mark I., Transcutaneous Electrical Nerve Stimulation (TENS): Research to support clinical practice, Oxford University Press, 2014 | + | Quell is wearable device developed in purpose to provide palliative treatment for people suffering from chronical pain. Quell is commercially available, thus customers don't need any prescription from a doctor. In present, it is the only palliative wearable, which is approved by FDA to be used during sleep. Quell wearable is presented by two technological improvements, which is OptiTherapy™ electrode and transcutaneous electrical nerve stimulation (TENS), method allowing sending electrical pulses into afferent nerves through the skin. The technologie of TENS is not new, but it was developed on knowledge about possibility to heal with electricity, which was described already in 18th century.<ref name="johnson14">Johnson, Mark I., Transcutaneous Electrical Nerve Stimulation (TENS): Research to support clinical practice, Oxford University Press, 2014</ref> Quell device was constructed in the beginning of 21th century, on the basis of knowledge about TENS.<ref name="science-behind-quell.pdf">Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf, (Retrieved Dec 15, 2015)</ref> its technology, marketing strategies and usefulness, regarding human enhancement and therapy. |
| − | + | ||
| Line 73: | Line 67: | ||
User have to buy accessories in addition like travel bag, spare cables, charger adapter, sports band<ref>Quell, Accessories, Online shop webpage: https://store.quellrelief.com/collections/accessories</ref>. And also two electrodes, user has to buy regularly, and which are currently available in two kinds of packages: one package of two electrodes for 29.95$, or three packages of two electrodes for 89.85$<ref>Quell, Electrodes, Online shop webpage: https://store.quellrelief.com/collections/electrodes</ref>. | User have to buy accessories in addition like travel bag, spare cables, charger adapter, sports band<ref>Quell, Accessories, Online shop webpage: https://store.quellrelief.com/collections/accessories</ref>. And also two electrodes, user has to buy regularly, and which are currently available in two kinds of packages: one package of two electrodes for 29.95$, or three packages of two electrodes for 89.85$<ref>Quell, Electrodes, Online shop webpage: https://store.quellrelief.com/collections/electrodes</ref>. | ||
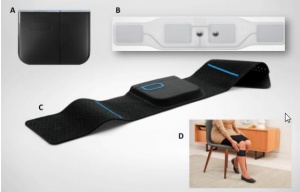
| − | [[File:Quell-components.jpg|thumbnail|A) A therapy pod, B) An electrode array, C) A Band, D) An example of usage on upper calf, place of effect<ref | + | [[File:Quell-components.jpg|thumbnail|A) A therapy pod, B) An electrode array, C) A Band, D) An example of usage on upper calf, place of effect<ref name="science-behind-quell.pdf"/>]] |
Regardless which part of body is in pain, Quell is always placed on upper calf (no matter which side). The reason is that there is better contact with nerves, on which are impulses sent to part of brainstem. After the devices is fastened around the calf, user needs only to press the button on it. The starter kit has inbuilt accelerometer which allows calibration of electrical intensity according to user's body, manually regulating is not needed. | Regardless which part of body is in pain, Quell is always placed on upper calf (no matter which side). The reason is that there is better contact with nerves, on which are impulses sent to part of brainstem. After the devices is fastened around the calf, user needs only to press the button on it. The starter kit has inbuilt accelerometer which allows calibration of electrical intensity according to user's body, manually regulating is not needed. | ||
| Line 79: | Line 73: | ||
For easier control it is possible to download Quell application for iOS<ref>Quell relief application available to download: https://itunes.apple.com/app/quell-relief/id972079954</ref> or Android<ref>Quell relief application available to download on GooglePlay: https://play.google.com/store/apps/details?id=com.neurometrix.quell</ref>. Company offers also [https://www.quellrelief.com/quell-user registration] of individual user's device, which will allow company databasis to collect measured data about user and treatment process (this registration is optional, though). | For easier control it is possible to download Quell application for iOS<ref>Quell relief application available to download: https://itunes.apple.com/app/quell-relief/id972079954</ref> or Android<ref>Quell relief application available to download on GooglePlay: https://play.google.com/store/apps/details?id=com.neurometrix.quell</ref>. Company offers also [https://www.quellrelief.com/quell-user registration] of individual user's device, which will allow company databasis to collect measured data about user and treatment process (this registration is optional, though). | ||
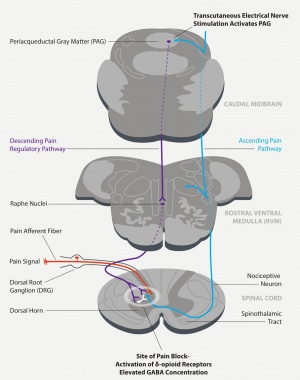
| − | Mechanism of the treatment lies in the following process: in electrode that stimulates clusters of afferent senzoric nerves and this | + | Mechanism of the treatment lies in the following process: in electrode that stimulates clusters of afferent senzoric nerves and this triggers inhibition of pain in brainstem. Inhibition comes up due to cascade neurotransmitter release. This descending process starts in periaqueductal gray matter, continuing to medulla and then into spinal cord (its dorsal horn).<ref>Introducing Quell™, a 100% drug free technology proven to fight pain: https://www.quellrelief.com/files/Quell%20HCP%201%20page%20(3).pdf</ref> Within-body-produced opioids, enkephalins, causes inhibition of pain signal transmission, while activate δ-opioid receptors. The described process is the core of reason why it is cleared as 100% drug free. More detailed description regarding TENS technology (not Quell in particular) is available in Johnson's book Transcutaneous Electrical Nerve Stimulation.<ref name="johnson14" /> |
Company also claims, that Quell is as twice stronger as previous TENS pain relieving products, thus it doesn't have to be used locally, but only on an upper calf.<ref>NeuroMetrix Investor statement: http://investor.neurometrix.com/releasedetail.cfm?releaseid=923300, December 15, 2015</ref> | Company also claims, that Quell is as twice stronger as previous TENS pain relieving products, thus it doesn't have to be used locally, but only on an upper calf.<ref>NeuroMetrix Investor statement: http://investor.neurometrix.com/releasedetail.cfm?releaseid=923300, December 15, 2015</ref> | ||
| Line 88: | Line 82: | ||
<!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | <!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | ||
| − | Quell wearable is device designated for treatment of chronic pain, nerve deseases and disrupted sleep.<ref>Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html</ref> It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief. | + | Quell wearable is device designated for treatment of chronic pain, nerve deseases and disrupted sleep.<ref name="company-overview">Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html, (Retrieved Dec 15, 2015)</ref> It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief. |
=== Company & People === | === Company & People === | ||
<!-- This is a list of important people behind the technology. Note: It is not necessary to list absolutely everyone. --> | <!-- This is a list of important people behind the technology. Note: It is not necessary to list absolutely everyone. --> | ||
| − | Neurometrix is company directed on developing health-care wearable technology with main effort on sleep disorders, chronical pain, and nerve deseases treatment. The company, having a seat in Waltham Massachusetts, was founded in 1996 as a spinoff of the Harvard-MIT partition of Health Sciences and Technology.<ref | + | Neurometrix is company directed on developing health-care wearable technology with main effort on sleep disorders, chronical pain, and nerve deseases treatment. The company, having a seat in Waltham Massachusetts, was founded in 1996 as a spinoff of the Harvard-MIT partition of Health Sciences and Technology.<ref name="company-overview"/><ref>NeuroMetrix Launches Quell™ Wearable Pain Relief Technology: http://www.businesswire.com/news/home/20150615005489/en/</ref> |
| − | Currently Neurometrix offers four products in total:<ref | + | Currently Neurometrix offers four products in total:<ref name="company-overview"/> |
* Quell™ - Wearable device for palliative purpose | * Quell™ - Wearable device for palliative purpose | ||
| Line 124: | Line 118: | ||
Important dates in history of TENS: | Important dates in history of TENS: | ||
| − | * 1759 - John Wesley described medical usage of electricity for sciatica, headache, gout, and kidney stones treatment.<ref | + | * 1759 - John Wesley described medical usage of electricity for sciatica, headache, gout, and kidney stones treatment.<ref name="johnson14"/> |
| − | * 1965 - Melzack and Wall proposed first conceptual model for mechanism which could lead to pain relief. They rationalized that harmful signals may be inhibited by peripheral afferents of large diameter.<ref | + | * 1965 - Melzack and Wall proposed first conceptual model for mechanism which could lead to pain relief. They rationalized that harmful signals may be inhibited by peripheral afferents of large diameter.<ref name="johnson14"/> |
| − | * 1970s - Developement of TENS (Transcutaneous electrical nerve stimulation) | + | * 1970s - Developement of TENS (Transcutaneous electrical nerve stimulation).<ref name="johnson14"/> |
| − | * 2007 - Johnson and Martinson - Largest meta-analysis of evidences about three times stronger pain relief than in placebo.<ref | + | * 2007 - Johnson and Martinson - Largest meta-analysis of evidences about three times stronger pain relief than in placebo.<ref name="johnson14"/> |
Important dates of Quell device history: | Important dates of Quell device history: | ||
| − | * 1996 - Establishment of Neurometrix | + | * 1996 - Establishment of Neurometrix.<ref name="company-overview"/> |
| − | * 2014 - FDA gives a clearance to release Quell wearable device. | + | * 2014 - FDA gives a clearance to release Quell wearable device.<ref name="shipping">NeuroMetrix Begins Shipping Quell Device, June 1, 2015: http://www.fdanews.com/articles/171428 (Retrieved Dec 15, 2015)</ref> |
== Ethical Issues == | == Ethical Issues == | ||
<!-- Provide detailed information (if possible or if available) about any ethical risks and related ethical topics or dilemmas in this section. ---> | <!-- Provide detailed information (if possible or if available) about any ethical risks and related ethical topics or dilemmas in this section. ---> | ||
| − | In present, there are no ethical issues known. | + | In present, there are no ethical issues known. Quell device's purpose is to provide lenitive care (and also it is FDA cleared). The device may help people from pain of unknown cause, and unkown way of treatment. This can be considered as positive ethical value. |
| + | |||
| + | Also no modification of human body is included except of enkephalins'stimulation, and any long-term consequences in human organism are not known. | ||
== Health Risks == | == Health Risks == | ||
<!-- Any related health issues, be it already discovered and covered in literature, or just speculative ones, should be described and properly cited in this section. --> | <!-- Any related health issues, be it already discovered and covered in literature, or just speculative ones, should be described and properly cited in this section. --> | ||
| − | Official statement from Neurometrix about side effects is that there are none[https://www.quellrelief.com/clinicians]. Usage is 100% drug free, with clearance by FDA (Food and Drug Administration) received in 2014. | + | Official statement from Neurometrix about side effects is that there are none[https://www.quellrelief.com/clinicians]. Usage is 100% drug free, with clearance by FDA (Food and Drug Administration) received in 2014.<ref name="shipping"/> |
| − | No side effects mentioned are mentioned. There are some contradictions that patients with cardiac pacemakers or with implanted defibrillator should not use Quell, neither people with implated metallic or electronic devices.<ref>Frequently Asked Questions by Clinicians: https://www.quellrelief.com/clinicians, December 15, 2015</ref> Patient is supposed to consult the using with a doctor. Despite | + | No side effects mentioned are mentioned. There are some contradictions that patients with cardiac pacemakers or with implanted defibrillator should not use Quell, neither people with implated metallic or electronic devices.<ref>Frequently Asked Questions by Clinicians: https://www.quellrelief.com/clinicians, December 15, 2015</ref> Patient is supposed to consult the using with a doctor. Despite the mentioned contradictions, Quell company says they have customers who have pacemakers and use the device succesfully (See picture). |
| − | [[File:Screenshot003|thumbnail|Quell's responses to the user's question, whether the device is contradicting to tachycardia. https://www.facebook.com/quellrelief/?fref=ts ]] | + | [[File:Screenshot003.jpg|thumbnail|Quell's responses to the user's question, whether the device is contradicting to tachycardia. https://www.facebook.com/quellrelief/?fref=ts, December 1, 2015]] |
On the other hand no medication doesn't restrict Quell-using.<ref>Quell relief official website, Clinicians: https://www.quellrelief.com/clinicians</ref> | On the other hand no medication doesn't restrict Quell-using.<ref>Quell relief official website, Clinicians: https://www.quellrelief.com/clinicians</ref> | ||
| Line 154: | Line 150: | ||
Nevertheless we should wait for some new quality scientific trials and longe to show if Quell device brings some currently unknown specific healthy risks. | Nevertheless we should wait for some new quality scientific trials and longe to show if Quell device brings some currently unknown specific healthy risks. | ||
| − | Speculatively, it might happen that palliative treatment will lead only to pain elimination but not causation cure, which could cause some grave injury. Let's consider, in a case of scoliosis, some physical workout could help to strenghten the back muscles and thus to stop a back pain. Pacients with any continual body-pain should take in consideration if there are any ways to cure cause of pain first. This discussion is based on some videos and comments of people using Quell just on scoliosis.<ref>Quell Wearable Pain Relief For Sciatica Review And Walkthrough, in: https://www.youtube.com/watch?v=BHI7PSxXbSw, | + | Speculatively, it might happen that palliative treatment will lead only to pain elimination but not causation cure, which could cause some grave injury. Let's consider, in a case of scoliosis, some physical workout could help to strenghten the back muscles and thus to stop a back pain. Pacients with any continual body-pain should take in consideration if there are any ways to cure cause of pain first. This discussion is based on some videos and comments of people using Quell just on scoliosis.<ref>Quell Wearable Pain Relief For Sciatica Review And Walkthrough, in: https://www.youtube.com/watch?v=BHI7PSxXbSw, (retrieved 12/8/2015)</ref> |
== Enhancement/Therapy/Treatment == | == Enhancement/Therapy/Treatment == | ||
| Line 165: | Line 161: | ||
* [https://en.wikipedia.org/wiki/Sciatica Sciatica] | * [https://en.wikipedia.org/wiki/Sciatica Sciatica] | ||
| − | However, diseases or illnesses are also identified as possible to be treated by TENS: Acute postoperative pain, primary dysmenorrhea, labor pain (which still remains controversial<ref>Sluka KA, Walsh D., Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness, in: Journal of Pain, 2003, p. 109-121</ref> ), post-stroke shoulder pain. | + | However, diseases or illnesses are also identified as possible to be treated by TENS: Acute postoperative pain, primary dysmenorrhea, labor pain (which still remains controversial<ref name="tens">Sluka KA, Walsh D., Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness, in: Journal of Pain, 2003, p. 109-121</ref> ), post-stroke shoulder pain. |
| − | Although Quell was not created primarily to enhance cognitive or physical condition in humans, it may have secondary impact on physical and mental health (e.g. emotional stability, better memory) due to stress release, better sleep periods, and ability to be more concentrated if pain disappears. As a result | + | Although Quell was not created primarily to enhance cognitive or physical condition in humans, it may have secondary impact on physical and mental health (e.g. emotional stability, better memory) due to stress release, better sleep periods, and ability to be more concentrated if pain disappears. As a result may be quality of life enhanced generally. |
== Public & Media Impact and Presentation == | == Public & Media Impact and Presentation == | ||
| Line 175: | Line 171: | ||
=== Company official promotion === | === Company official promotion === | ||
| − | Products of Neurometrix are presented on the official Neurometrix websites, and also each of them has its own [https://www.quellrelief.com/ webpage]. It is question to which extent can be this website trustworthy or customer-friendly, regarding following reasons. | + | Products of Neurometrix are presented on the official Neurometrix websites, and also each of them has its own [https://www.quellrelief.com/ webpage]. It is question to which extent can be this website trustworthy or customer-friendly, regarding following reasons. It is untransparency of information: It takes longer time to find technical parameters or principles of mechanisms and useful information. Next reason is absence of ethical aspect of Quell device. Customers will not see what does company thinks about such an issue. The last reason is that website lacks references to independent scientific studies which would criticaly evaluate the device's mechanism. |
| − | There is also official launch [https://player.vimeo.com/video/116014236/ video] available on the [https://www.quellrelief.com/ Quell product website]. In | + | There is also official launch [https://player.vimeo.com/video/116014236/ video] available on the [https://www.quellrelief.com/ Quell product website]. In 2014, [https://www.facebook.com/quellrelief/?fref=ts Quell official facebook page] has been launched, and it's still active. Also its administrators shows some will to answer on users' questions and to share new information with them. Quell has also its own [https://www.youtube.com/channel/UC0om7ZbCA8mdchvTPQ6BEdg youtube channel], however, it contains only one video and otherwise this channel seems to be currently not active. |
=== Other mentions === | === Other mentions === | ||
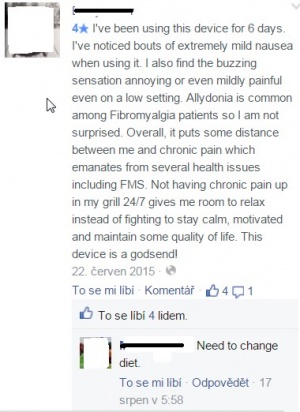
| + | [[File:Comment.jpg|thumbnail|right|(Img. 1) Pacient's experience with Quell device. He claims he feels improvement but also nausea. [https://www.facebook.com/quellrelief/?fref=ts Official facebook page]]] | ||
| − | There are some unofficial pages discussing Quell relief in point of view of business, technology, or healthcare. In their articles, it seems they use promotional rhetoric, without mentioning no scientificaly verified information | + | [[File:Partial improvement.jpg|thumbnail|right|Customer is satisfied with the Quell device, but claims that pain relief is not complete.<ref>Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015</ref>]] |
| + | |||
| + | [[File:Not satisfied.jpg|thumbnail|right|Customer is unsatisfied with Quell, although his disorder is one of those that can be treaten by Quell. <ref>Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015</ref>]] | ||
| + | |||
| + | There are some unofficial pages discussing Quell relief in point of view of business, technology, or healthcare. In their articles, it seems they use promotional rhetoric, without mentioning no scientificaly verified information. For example [http://www.humavox.com/blog/wearable-week-quell-pain-relief-wearable/ here] in the article "Wearable of the Week: Quell Drug-Free Pain Relief Wearable" on Humavox). | ||
[http://www.wxyz.com/news/wearable-quell-could-provide-pain-relief Could wearable Quell provide pain relief?] on Detroit TV-channel WXYZ. | [http://www.wxyz.com/news/wearable-quell-could-provide-pain-relief Could wearable Quell provide pain relief?] on Detroit TV-channel WXYZ. | ||
| Line 195: | Line 196: | ||
* [https://www.youtube.com/watch?v=9vbLGVXIfvI Could wearable Quell provide pain relief? - WXYZ-TV Detroit | Channel 7, 9. 1. 2015] | * [https://www.youtube.com/watch?v=9vbLGVXIfvI Could wearable Quell provide pain relief? - WXYZ-TV Detroit | Channel 7, 9. 1. 2015] | ||
| − | The internet medical journal [http://www.medgadget.com/2015/08/hands-on-with-quell-wearable-pain-relief-device.html MedGadget] has released an independent review of Quell: | + | The internet medical journal [http://www.medgadget.com/2015/08/hands-on-with-quell-wearable-pain-relief-device.html MedGadget] has released an independent review of Quell: |
"''Likes'': | "''Likes'': | ||
| Line 209: | Line 210: | ||
* ''Did not experience significant pain relief after two weeks of use'' | * ''Did not experience significant pain relief after two weeks of use'' | ||
* ''Users with better success might have difficulty finding the optimal setting due to limited programming options on both the device and app'' | * ''Users with better success might have difficulty finding the optimal setting due to limited programming options on both the device and app'' | ||
| − | * ''You’ll need to spend $30 on extra electrode strips'' " | + | * ''You’ll need to spend $30 on extra electrode strips'' "<ref name="hands on">Hands-on with Quell Wearable Pain Relief Device: http://www.medgadget.com/2015/08/hands-on-with-quell-wearable-pain-relief-device.html, (Retrieved Dec 15, 2015)</ref> |
| − | MedGadget's conclusion is that "''We unfortunately failed to find significant pain relief after using this $250 device for a few weeks, but much more testing is needed before we can come to a definite conclusion.''"<ref | + | MedGadget's conclusion is that "''We unfortunately failed to find significant pain relief after using this $250 device for a few weeks, but much more testing is needed before we can come to a definite conclusion.''"<ref name="hands on"/> However, MedGadget does not provide any specific data to support their claims about the disadvantages. |
| + | Besides another official presentation, many individual users share their experiences with the device on their blogs or Facebook posts, comments, ratings etc. Blog-contributors describe using Quell mostly as positive experience. People are not satisfied with are unincluded insurance, hardly avalailable information, or expensive electrode which needs to be changed more than it is officially stated and other inconsistencies. For example user claims "...''the replacement electrodes cost $30 for 2 ($15 each) and each one is supposed to last 2 weeks, however, I used my first one for exactly 4 weeks before it stopped working as well.''"<ref>Comment placed by Gina McNabb, in: http://www.painnewsnetwork.org/stories/2015/7/22/wear-tear-care-the-quell-pain-relief-device, 12/8/2015</ref> | ||
| − | + | Individual user's manual- or testimony-like videos can be found as well. There the user confess no affiliation with the company: [https://www.youtube.com/watch?v=BHI7PSxXbSw Quell Wearable Pain Relief For Sciatica Review And Walkthrough, 7. 7. 2015] | |
| − | + | ||
| − | + | Only health issue found was nausea, although pacient claimed pain reduction (see picture below) - this claim is actually the only one which neither Quell site, nor dr. Shai Gozani has responded to. However, there's no evidence if it is really side-effect of Quell usage. | |
| + | |||
| + | People also comment intensity of pain reduction. Results may vary from fully satisfying treatment over partial improvement to no advances in pain relief at all. (see pictures from the youtube chat<ref>Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015</ref>) | ||
| + | |||
| + | Below are examples of various blogs with articles dealing with Quell: | ||
* [http://www.footdoctorsnj.com/blog/2015/09/30/1709/ Quell: Relief From Chronic Pain] | * [http://www.footdoctorsnj.com/blog/2015/09/30/1709/ Quell: Relief From Chronic Pain] | ||
| Line 226: | Line 231: | ||
* [http://www.painnewsnetwork.org/stories/2015/7/22/wear-tear-care-the-quell-pain-relief-device Wear, Tear & Care: The Quell Pain Relief Device] | * [http://www.painnewsnetwork.org/stories/2015/7/22/wear-tear-care-the-quell-pain-relief-device Wear, Tear & Care: The Quell Pain Relief Device] | ||
| − | |||
| − | |||
== Public Policy == | == Public Policy == | ||
<!-- Information related to any regulations (law, patents, ISOs, government recommendations and so on.) --> | <!-- Information related to any regulations (law, patents, ISOs, government recommendations and so on.) --> | ||
| − | Quell received clearance from [http:// | + | Quell received clearance from [http://www.fda.gov Food and Drug Administration], that is federal agency as part of Department of Health and Human Services in the USA. |
| − | Quell is kept under regulation number: 882.5890, and it is identified as "A transcutaneous electrical nerve stimulator for pain relief is a device used to apply an electrical current to electrodes on a patient's skin to treat pain".<ref>Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890</ref> Further, it is classified with a "Class II (performance standards)".<ref | + | Quell is kept under regulation number: 882.5890, and it is identified as "A transcutaneous electrical nerve stimulator for pain relief is a device used to apply an electrical current to electrodes on a patient's skin to treat pain".<ref name="code">Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890</ref> Further, it is classified with a "Class II (performance standards)".<ref name="code"/> The latter category features most of medical devices (FDA stated 43%), including e. g. wheelchairs or pregnancy test kits.<ref>Medical device classification list on: http://www.fda.gov/MedicalDevices/ResourcesforYou/Consumers/ucm142523.htm</ref> |
== Related Technologies, Project or Scientific Research == | == Related Technologies, Project or Scientific Research == | ||
| Line 238: | Line 241: | ||
=== WINS Technology === | === WINS Technology === | ||
| − | Neurometrix says they use so called Wearable Intensive Nerve Stimulation (WINS), however, no studies are provided to explain its mechanisms. | + | Neurometrix says they use so called Wearable Intensive Nerve Stimulation (WINS),<ref>Main page of Quell relief: https://www.quellrelief.com/, (Retrieved Dec 15, 2015)</ref> however, no studies are provided to explain its mechanisms. |
=== TENS Research === | === TENS Research === | ||
| − | + | There is only one paper about Quell principles right on the official Quell website,<ref name="science-behind-quell.pdf"/> but this doesn't contain necessities of independent scientific study. Although there are no other studies dealing with Quell product specifically, an independent research about transcutaneous electric nervous stimulation (TENS) was conducted. This study appear not to be unambiguous regarding good efficacy of TENS in treatment. Here follow few works engaged in TENS technology and its influence on different deseases. | |
| − | + | ||
| − | + | ||
| − | + | Sluka and Walsh<ref name="tens"/> summarize existing researches of TENS, and shows couple of results of scientific trials, and speak about equivocal effectiveness remaining due to low number of randomized and controlled trials. Sluka and Walsh quote that different trials for one disease may have contrasting results, as in case with treating labor pain.<ref name="tens"/> | |
| − | + | Mark I. Johnson is author of a book supporting TENS technology.<ref name="johnson14"/> | |
== References == | == References == | ||
Latest revision as of 00:15, 28 January 2016
| QUELL RELIEF | |
|---|---|

|
|
| Category | Limb-mounted |
| Developer | NeuroMetrix |
| Announced | Mar 2015[1] |
| Released | Consumers: May 2015 [2] |
| Price | 249 USD |
| Weight | 62 g |
| Controls | smartphone |
| Standalone[3] | |
| https://www.quellrelief.com/product | |
Quell is wearable device developed in purpose to provide palliative treatment for people suffering from chronical pain. Quell is commercially available, thus customers don't need any prescription from a doctor. In present, it is the only palliative wearable, which is approved by FDA to be used during sleep. Quell wearable is presented by two technological improvements, which is OptiTherapy™ electrode and transcutaneous electrical nerve stimulation (TENS), method allowing sending electrical pulses into afferent nerves through the skin. The technologie of TENS is not new, but it was developed on knowledge about possibility to heal with electricity, which was described already in 18th century.[4] Quell device was constructed in the beginning of 21th century, on the basis of knowledge about TENS.[5] its technology, marketing strategies and usefulness, regarding human enhancement and therapy.
Contents
Main characteristics
The basic product a customer can buy, called Quell wearable pain relief - starter kit[6], includes one piece of Quell device, adjustable sports band, charger cable, and charger adapter, and two 2 electrodes (company claims one month supply). Manual is available to download in the Quell-user category, in English, and Spanish language.
User have to buy accessories in addition like travel bag, spare cables, charger adapter, sports band[7]. And also two electrodes, user has to buy regularly, and which are currently available in two kinds of packages: one package of two electrodes for 29.95$, or three packages of two electrodes for 89.85$[8].
Regardless which part of body is in pain, Quell is always placed on upper calf (no matter which side). The reason is that there is better contact with nerves, on which are impulses sent to part of brainstem. After the devices is fastened around the calf, user needs only to press the button on it. The starter kit has inbuilt accelerometer which allows calibration of electrical intensity according to user's body, manually regulating is not needed.
For easier control it is possible to download Quell application for iOS[9] or Android[10]. Company offers also registration of individual user's device, which will allow company databasis to collect measured data about user and treatment process (this registration is optional, though).
Mechanism of the treatment lies in the following process: in electrode that stimulates clusters of afferent senzoric nerves and this triggers inhibition of pain in brainstem. Inhibition comes up due to cascade neurotransmitter release. This descending process starts in periaqueductal gray matter, continuing to medulla and then into spinal cord (its dorsal horn).[11] Within-body-produced opioids, enkephalins, causes inhibition of pain signal transmission, while activate δ-opioid receptors. The described process is the core of reason why it is cleared as 100% drug free. More detailed description regarding TENS technology (not Quell in particular) is available in Johnson's book Transcutaneous Electrical Nerve Stimulation.[4]
Company also claims, that Quell is as twice stronger as previous TENS pain relieving products, thus it doesn't have to be used locally, but only on an upper calf.[12]
Purpose
Quell wearable is device designated for treatment of chronic pain, nerve deseases and disrupted sleep.[14] It works on principle of transcutaneous electrical nerve stimulation, which causes release of endogenous opiates affecting pain relief.
Company & People
Neurometrix is company directed on developing health-care wearable technology with main effort on sleep disorders, chronical pain, and nerve deseases treatment. The company, having a seat in Waltham Massachusetts, was founded in 1996 as a spinoff of the Harvard-MIT partition of Health Sciences and Technology.[14][15]
Currently Neurometrix offers four products in total:[14]
- Quell™ - Wearable device for palliative purpose
- SENSUS® - Therapy device available on prescription. It is based on TENS technology also for pain relief without any drug usage.
- DPNCheck® - Nerve conduction test for evaluating systemic neuropathies such as diabetic peripheral neuropathy (DPN), its detection and monitoring.
- ADVANCE™ NCS System - apparatus meant to assessing neuropathy diagnosis.
Directors of Neurometrix[16]:
- Shai N. Gozani, M.D., Ph.D.
- Allen Hinkle, M.D.
- David E. Goodman, M.D
- Timothy R. Surgenor
- Nancy E. Katz
- David Van Avermaete
Important Dates
Important dates in history of TENS:
- 1759 - John Wesley described medical usage of electricity for sciatica, headache, gout, and kidney stones treatment.[4]
- 1965 - Melzack and Wall proposed first conceptual model for mechanism which could lead to pain relief. They rationalized that harmful signals may be inhibited by peripheral afferents of large diameter.[4]
- 1970s - Developement of TENS (Transcutaneous electrical nerve stimulation).[4]
- 2007 - Johnson and Martinson - Largest meta-analysis of evidences about three times stronger pain relief than in placebo.[4]
Important dates of Quell device history:
- 1996 - Establishment of Neurometrix.[14]
- 2014 - FDA gives a clearance to release Quell wearable device.[17]
Ethical Issues
In present, there are no ethical issues known. Quell device's purpose is to provide lenitive care (and also it is FDA cleared). The device may help people from pain of unknown cause, and unkown way of treatment. This can be considered as positive ethical value.
Also no modification of human body is included except of enkephalins'stimulation, and any long-term consequences in human organism are not known.
Health Risks
Official statement from Neurometrix about side effects is that there are none[1]. Usage is 100% drug free, with clearance by FDA (Food and Drug Administration) received in 2014.[17] No side effects mentioned are mentioned. There are some contradictions that patients with cardiac pacemakers or with implanted defibrillator should not use Quell, neither people with implated metallic or electronic devices.[18] Patient is supposed to consult the using with a doctor. Despite the mentioned contradictions, Quell company says they have customers who have pacemakers and use the device succesfully (See picture).
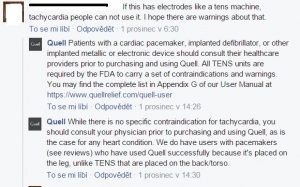
On the other hand no medication doesn't restrict Quell-using.[19]
However, there are another information sources which discuss TENS technology. For example they claim, that TENS method is surely inexpensive and safe, but there are risk with using electrodes on damaged skin, or over area of malignancy,[20] or it could trigger a seizure in epileptic patient in case that electrodes will be put near patient's head or neck.[21] Regarding these risks, most of them patients may avoid as Quell is used only on an upper calf. Nevertheless we should wait for some new quality scientific trials and longe to show if Quell device brings some currently unknown specific healthy risks.
Speculatively, it might happen that palliative treatment will lead only to pain elimination but not causation cure, which could cause some grave injury. Let's consider, in a case of scoliosis, some physical workout could help to strenghten the back muscles and thus to stop a back pain. Pacients with any continual body-pain should take in consideration if there are any ways to cure cause of pain first. This discussion is based on some videos and comments of people using Quell just on scoliosis.[22]
Enhancement/Therapy/Treatment
Quell wearable device has primarily therapeutical function. Company states it was developed on purpose to palliate chronical pain which basically has origin in these four diseases:
However, diseases or illnesses are also identified as possible to be treated by TENS: Acute postoperative pain, primary dysmenorrhea, labor pain (which still remains controversial[23] ), post-stroke shoulder pain.
Although Quell was not created primarily to enhance cognitive or physical condition in humans, it may have secondary impact on physical and mental health (e.g. emotional stability, better memory) due to stress release, better sleep periods, and ability to be more concentrated if pain disappears. As a result may be quality of life enhanced generally.
Public & Media Impact and Presentation
Regarding business interests in presenting this product in public, and the fact that internet is anonymous space where we have no clue who is author of opinions or arguments, readers should consider that information found may be false or may be results of so called trolling. This concerns especially risks of possible health risks being hidden by promotion companies and institutions, further it could be individual people who could be paid for competitive malignment, or otherwise people praising a product untruthfully, or there may be people who just want to troll and thwart other project.
Company official promotion
Products of Neurometrix are presented on the official Neurometrix websites, and also each of them has its own webpage. It is question to which extent can be this website trustworthy or customer-friendly, regarding following reasons. It is untransparency of information: It takes longer time to find technical parameters or principles of mechanisms and useful information. Next reason is absence of ethical aspect of Quell device. Customers will not see what does company thinks about such an issue. The last reason is that website lacks references to independent scientific studies which would criticaly evaluate the device's mechanism.
There is also official launch video available on the Quell product website. In 2014, Quell official facebook page has been launched, and it's still active. Also its administrators shows some will to answer on users' questions and to share new information with them. Quell has also its own youtube channel, however, it contains only one video and otherwise this channel seems to be currently not active.
Other mentions

There are some unofficial pages discussing Quell relief in point of view of business, technology, or healthcare. In their articles, it seems they use promotional rhetoric, without mentioning no scientificaly verified information. For example here in the article "Wearable of the Week: Quell Drug-Free Pain Relief Wearable" on Humavox).
Could wearable Quell provide pain relief? on Detroit TV-channel WXYZ.
Many advertising videos and testimonials are to be found on youtube. Here are some examples of them:
The internet medical journal MedGadget has released an independent review of Quell:
"Likes:
- Utilizes scientifically-validated and medically-safe therapy for pain relief
- Aesthetically-pleasing and portable design
- Connects to an iPhone via Bluetooth to track sessions
Dislikes:
- Did not experience significant pain relief after two weeks of use
- Users with better success might have difficulty finding the optimal setting due to limited programming options on both the device and app
- You’ll need to spend $30 on extra electrode strips "[26]
MedGadget's conclusion is that "We unfortunately failed to find significant pain relief after using this $250 device for a few weeks, but much more testing is needed before we can come to a definite conclusion."[26] However, MedGadget does not provide any specific data to support their claims about the disadvantages.
Besides another official presentation, many individual users share their experiences with the device on their blogs or Facebook posts, comments, ratings etc. Blog-contributors describe using Quell mostly as positive experience. People are not satisfied with are unincluded insurance, hardly avalailable information, or expensive electrode which needs to be changed more than it is officially stated and other inconsistencies. For example user claims "...the replacement electrodes cost $30 for 2 ($15 each) and each one is supposed to last 2 weeks, however, I used my first one for exactly 4 weeks before it stopped working as well."[27]
Individual user's manual- or testimony-like videos can be found as well. There the user confess no affiliation with the company: Quell Wearable Pain Relief For Sciatica Review And Walkthrough, 7. 7. 2015
Only health issue found was nausea, although pacient claimed pain reduction (see picture below) - this claim is actually the only one which neither Quell site, nor dr. Shai Gozani has responded to. However, there's no evidence if it is really side-effect of Quell usage.
People also comment intensity of pain reduction. Results may vary from fully satisfying treatment over partial improvement to no advances in pain relief at all. (see pictures from the youtube chat[28])
Below are examples of various blogs with articles dealing with Quell:
Public Policy
Quell received clearance from Food and Drug Administration, that is federal agency as part of Department of Health and Human Services in the USA. Quell is kept under regulation number: 882.5890, and it is identified as "A transcutaneous electrical nerve stimulator for pain relief is a device used to apply an electrical current to electrodes on a patient's skin to treat pain".[29] Further, it is classified with a "Class II (performance standards)".[29] The latter category features most of medical devices (FDA stated 43%), including e. g. wheelchairs or pregnancy test kits.[30]
Related Technologies, Project or Scientific Research
WINS Technology
Neurometrix says they use so called Wearable Intensive Nerve Stimulation (WINS),[31] however, no studies are provided to explain its mechanisms.
TENS Research
There is only one paper about Quell principles right on the official Quell website,[5] but this doesn't contain necessities of independent scientific study. Although there are no other studies dealing with Quell product specifically, an independent research about transcutaneous electric nervous stimulation (TENS) was conducted. This study appear not to be unambiguous regarding good efficacy of TENS in treatment. Here follow few works engaged in TENS technology and its influence on different deseases.
Sluka and Walsh[23] summarize existing researches of TENS, and shows couple of results of scientific trials, and speak about equivocal effectiveness remaining due to low number of randomized and controlled trials. Sluka and Walsh quote that different trials for one disease may have contrasting results, as in case with treating labor pain.[23]
Mark I. Johnson is author of a book supporting TENS technology.[4]
References
- ↑ NeuroMetrix Launches Indiegogo Campaign for Quell™ Wearable Pain Relief Technology: http://investor.neurometrix.com/releasedetail.cfm?ReleaseID=899167, December 15, 2015
- ↑ NeuroMetrix Announces First Shipments of Quell™ Wearable Pain Relief Device: http://investor.neurometrix.com/releasedetail.cfm?releaseid=912945, December 15, 2015
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Johnson, Mark I., Transcutaneous Electrical Nerve Stimulation (TENS): Research to support clinical practice, Oxford University Press, 2014
- ↑ 5.0 5.1 5.2 Science Behind Quell™ Wearable Pain Relief Technology for Treatment of Chronic Pain: https://www.quellrelief.com/files/science-behind-quell.pdf, (Retrieved Dec 15, 2015)
- ↑ Quell wearable pain relief, starter kit, online shop webpage: https://store.quellrelief.com/products/quell-wearable-pain-relief
- ↑ Quell, Accessories, Online shop webpage: https://store.quellrelief.com/collections/accessories
- ↑ Quell, Electrodes, Online shop webpage: https://store.quellrelief.com/collections/electrodes
- ↑ Quell relief application available to download: https://itunes.apple.com/app/quell-relief/id972079954
- ↑ Quell relief application available to download on GooglePlay: https://play.google.com/store/apps/details?id=com.neurometrix.quell
- ↑ Introducing Quell™, a 100% drug free technology proven to fight pain: https://www.quellrelief.com/files/Quell%20HCP%201%20page%20(3).pdf
- ↑ NeuroMetrix Investor statement: http://investor.neurometrix.com/releasedetail.cfm?releaseid=923300, December 15, 2015
- ↑ Introducing Quell, a 100% drug free technology proven to fight pain: https://www.quellrelief.com/files/Quell%20HCP%201%20page%20(3).pdf
- ↑ 14.0 14.1 14.2 14.3 Neurometrix, Company overview: http://www.neurometrix.com/about-neurometrix/company-overview.html, (Retrieved Dec 15, 2015)
- ↑ NeuroMetrix Launches Quell™ Wearable Pain Relief Technology: http://www.businesswire.com/news/home/20150615005489/en/
- ↑ Neurometrix, Board of directors: http://www.neurometrix.com/about-neurometrix/board-of-directors.html
- ↑ 17.0 17.1 NeuroMetrix Begins Shipping Quell Device, June 1, 2015: http://www.fdanews.com/articles/171428 (Retrieved Dec 15, 2015)
- ↑ Frequently Asked Questions by Clinicians: https://www.quellrelief.com/clinicians, December 15, 2015
- ↑ Quell relief official website, Clinicians: https://www.quellrelief.com/clinicians
- ↑ Searle, R.D., Bennett M.I., Johnson M.I., Callin S., Radford H., Letter to editor: transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. Palliat Med, 2008; 22 : 878 - 9
- ↑ Jones, Iain, Johnson Mark I., Transcutaneous electrical nerve stimulation, 2009
- ↑ Quell Wearable Pain Relief For Sciatica Review And Walkthrough, in: https://www.youtube.com/watch?v=BHI7PSxXbSw, (retrieved 12/8/2015)
- ↑ 23.0 23.1 23.2 Sluka KA, Walsh D., Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness, in: Journal of Pain, 2003, p. 109-121
- ↑ Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015
- ↑ Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015
- ↑ 26.0 26.1 Hands-on with Quell Wearable Pain Relief Device: http://www.medgadget.com/2015/08/hands-on-with-quell-wearable-pain-relief-device.html, (Retrieved Dec 15, 2015)
- ↑ Comment placed by Gina McNabb, in: http://www.painnewsnetwork.org/stories/2015/7/22/wear-tear-care-the-quell-pain-relief-device, 12/8/2015
- ↑ Quell Wearable Pain Relief For Sciatica Review And Walkthrough: https://www.youtube.com/watch?v=BHI7PSxXbSw, 7. 7. 2015
- ↑ 29.0 29.1 Code of Federal Regulations, Title 21, Volume 8, 21CFR882.5890 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRsearch.cfm?FR=882.5890
- ↑ Medical device classification list on: http://www.fda.gov/MedicalDevices/ResourcesforYou/Consumers/ucm142523.htm
- ↑ Main page of Quell relief: https://www.quellrelief.com/, (Retrieved Dec 15, 2015)