Difference between revisions of "Cefaly"
(→Health Risks: pic) |
(changed categories) |
||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 13: | Line 13: | ||
<tr> | <tr> | ||
<th scope="row">Category</th> | <th scope="row">Category</th> | ||
| − | <td>[[Is categorized as:: | + | <td>[[Is categorized as::Head-mounted devices]]</td> |
</tr> | </tr> | ||
| Line 23: | Line 23: | ||
<tr> | <tr> | ||
<th scope="row">Announced</th> | <th scope="row">Announced</th> | ||
| − | <td>[[Announced in:: | + | <td>[[Announced in::2014]]<ref>FDA approves device to treat migraine headaches: http://articles.latimes.com/2014/mar/11/science/la-sci-sn-fda-approves-device-to-prevent-migraine-headaches-20140311</ref></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
<th scope="row">Released</th> | <th scope="row">Released</th> | ||
| − | <td>Consumers: [[Released for consumers:: | + | <td>Consumers: [[Released for consumers::2015]]<ref>Cefaly Technology release: http://www.biospace.com/News/cefaly-technology-release-10000-u-s-migraine/361631 (retrieved Jan 28, 2016)</ref></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
<th scope="row">Price</th> | <th scope="row">Price</th> | ||
| − | <td>[[Costs:: | + | <td>[[Costs::295]] USD <ref name="CSet">Cefaly Set on the Cefaly official online-shop: http://www.cefalymedical.com/shop/traitement-migraine/english_a/set-cefaly.html (retrieved Jan 28, 2016)</ref></td> |
</tr> | </tr> | ||
| Line 39: | Line 39: | ||
<tr> | <tr> | ||
<th scope="row">Weight</th> | <th scope="row">Weight</th> | ||
| − | <td>[[Weights::]] g</td> | + | <td>[[Weights::45.4]] g <ref>Cefaly® Anti-migrain Device: http://www.amazon.co.uk/Cefaly-Cefaly%C2%AE-Anti-migraine-Device/dp/B009VPAS0M (retrieved Jan 28, 2016)</ref></td> |
</tr> | </tr> | ||
| Line 64: | Line 64: | ||
</table> | </table> | ||
| + | |||
| + | Cefaly is head-mounted wearable device developed in purpose to treat and prevent migraine. | ||
<!-- Very brief description of the technology. This section should contain the main information about the subject. It's good to leave this bit as the last one and use the information provided in greater detail in later sections.--> | <!-- Very brief description of the technology. This section should contain the main information about the subject. It's good to leave this bit as the last one and use the information provided in greater detail in later sections.--> | ||
| Line 69: | Line 71: | ||
== Main characteristics == | == Main characteristics == | ||
<!-- This section should describe the technology in more detail. Here should be information about the used hardware and software, available features, chemical composition and so on, provided that they are available. Second half of this section should offer information on history of the technology. When it was created, unveiled, developed, announced to the public or when it was available to purchase. Anything related to the technology that can be pinpointed to a certain date should be in this section together with relevant commentary.--> | <!-- This section should describe the technology in more detail. Here should be information about the used hardware and software, available features, chemical composition and so on, provided that they are available. Second half of this section should offer information on history of the technology. When it was created, unveiled, developed, announced to the public or when it was available to purchase. Anything related to the technology that can be pinpointed to a certain date should be in this section together with relevant commentary.--> | ||
| + | The Cefaly neurostimulator has two forms according to position of the electrode:<ref name="2types">Two types of electrodes: http://www.cefaly.com/en/cefaly-electrodes (retrieved Jan 28, 2016)</ref> | ||
| + | |||
| + | 1. Forehead electrode - device consists of the headband with the electrode built in. The electrode impacts through the forehead. The whole headband device is available for 295 €<ref name="CSet"/>. However, customer can buy one package containing three electrodes for 19 €<ref name="2types"/>. | ||
| + | |||
| + | 2. Occipital electrode - inbuilt in the "Arnold kit". Its name comes from Arnold (Occipital) neuralgia. The electrode is placed on occipital bone with the assistace of the special headband. It is available for 69 €.<ref name="2types"/> | ||
| + | [[File:Occipitale-electrode.png|thumbnail|right|Occipital electrode called "Arnold kit" meant to treat occipital neuralgia<ref>Occipital neuralgia on wikipedia: https://en.wikipedia.org/wiki/Occipital_neuralgia (retrieved, Jan 28, 2016)</ref>.<ref name="2types"/>]] | ||
| + | |||
| + | Official website provides the manual with information how to use these products.<ref>The Electrodes user manual: http://www.cefaly.com/en/how-to-use-it (retrieved Jan 28, 2016)</ref> | ||
| + | |||
| + | In the UK and Ireland, The company also offers Cefaly to rent for 49 € per 2-month trial period. This is possible only on condition of paying the 295 €. If customer is dissatisfied, the company will pay him/her back 246 €.<ref>TRYING CEFALY on Cefaly official online-shop: http://www.cefaly.com/en/cefaly-shop (retrieved Jan 28, 2016)</ref> | ||
| + | |||
=== Purpose === | === Purpose === | ||
<!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | <!-- This is a very short description of the technology's purpose. What will it be doing, for what goal was it created, how it modifies human cognition. Keep this as brief as possible. --> | ||
| − | + | The electrode sends electrical impulses through the skin to branches of the trigeminal nerve. | |
=== Company & People === | === Company & People === | ||
| Line 95: | Line 108: | ||
Right on the official page of Cefaly side effects are stated.[http://www.cefaly.us/en/safety] Company claims, that side effects appear in 4,3% of patients, mentioning most common as intolerance to the feeling of Cefaly on the forehead (1.25%), sensation of fatigue during and after the session (0.65%), headache after one session (0.52%), or irritation of the skin on the forehead (0.22%). | Right on the official page of Cefaly side effects are stated.[http://www.cefaly.us/en/safety] Company claims, that side effects appear in 4,3% of patients, mentioning most common as intolerance to the feeling of Cefaly on the forehead (1.25%), sensation of fatigue during and after the session (0.65%), headache after one session (0.52%), or irritation of the skin on the forehead (0.22%). | ||
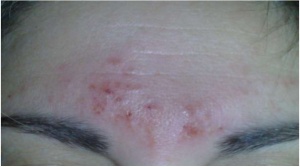
| + | Besides official statements, there is a study focused on Cefaly users' satisfaction with the treatment.<ref name="safety">Magis D., Sava S., d’Elia T. S., Baschi R., Schoenen J., Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177534/</ref> | ||
| − | [[File:Irritation Cefaly.jpg|thumbnail|Skin irritated probably | + | |
| + | [[File:Irritation Cefaly.jpg|thumbnail|Skin irritated probably by the electrode gel containing acrylate.<ref name="safety"/>]] | ||
== Enhancement/Therapy/Treatment == | == Enhancement/Therapy/Treatment == | ||
| Line 102: | Line 117: | ||
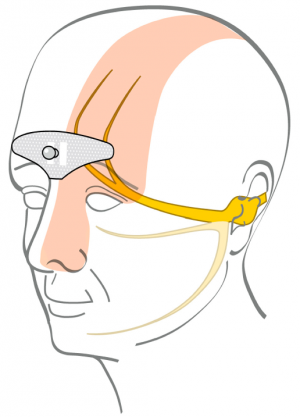
| − | [[File:Cefaly electrode effect.png|thumbnail|Electrode sends electric current through skin and affects trigeminal nerve. | + | [[File:Cefaly electrode effect.png|thumbnail|Electrode sends electric current through skin and affects trigeminal nerve.<ref name="safety"/>]] |
== Public & Media Impact and Presentation == | == Public & Media Impact and Presentation == | ||
| Line 110: | Line 125: | ||
== Public Policy == | == Public Policy == | ||
<!-- Information related to any regulations (law, patents, ISOs, government recommendations and so on.) --> | <!-- Information related to any regulations (law, patents, ISOs, government recommendations and so on.) --> | ||
| + | |||
| + | [http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm Announcement about approving migraine treat] | ||
| + | |||
| + | [http://www.accessdata.fda.gov/cdrh_docs/pdf12/K122566.pdf Memo clearance] | ||
== Related Technologies, Project or Scientific Research == | == Related Technologies, Project or Scientific Research == | ||
| Line 116: | Line 135: | ||
== References == | == References == | ||
| − | + | ||
| + | [[Category:External Hardware or Software]] | ||
| + | [[Category:Electronic and Other Devices]] | ||
[[Category:Wearables]] | [[Category:Wearables]] | ||
| − | [[Category:Head Mounted | + | [[Category:Head Mounted Devices]] |
| − | [[Category: | + | [[Category:Other Head-mounted Devices]] |
| − | + | [[Category:Brain Stimulation]] | |
Latest revision as of 08:49, 22 March 2016
| CEFALY | |
|---|---|

|
|
| Category | Head-mounted devices |
| Developer | STX-Med |
| Announced | 2014[1] |
| Released | Consumers: 2015[2] |
| Price | 295 USD [3] |
| Weight | 45.4 g [4] |
| Dimensions | mm |
| Controls | smartphone |
| Standalone[5] | |
| http://www.cefalytechnology.com/en/company | |
| http://www.cefalytechnology.com/en/products | |
Cefaly is head-mounted wearable device developed in purpose to treat and prevent migraine.
Contents
Main characteristics
The Cefaly neurostimulator has two forms according to position of the electrode:[6]
1. Forehead electrode - device consists of the headband with the electrode built in. The electrode impacts through the forehead. The whole headband device is available for 295 €[3]. However, customer can buy one package containing three electrodes for 19 €[6].
2. Occipital electrode - inbuilt in the "Arnold kit". Its name comes from Arnold (Occipital) neuralgia. The electrode is placed on occipital bone with the assistace of the special headband. It is available for 69 €.[6]
Official website provides the manual with information how to use these products.[8]
In the UK and Ireland, The company also offers Cefaly to rent for 49 € per 2-month trial period. This is possible only on condition of paying the 295 €. If customer is dissatisfied, the company will pay him/her back 246 €.[9]
Purpose
The electrode sends electrical impulses through the skin to branches of the trigeminal nerve.
Company & People
STX-Med company
Founders:
Pierre Rigaux
Pierre-Yves Muller
Important Dates
2004 - Foundation of STX-Med, which was later named Cefaly.[1]
Ethical Issues
Health Risks
Right on the official page of Cefaly side effects are stated.[2] Company claims, that side effects appear in 4,3% of patients, mentioning most common as intolerance to the feeling of Cefaly on the forehead (1.25%), sensation of fatigue during and after the session (0.65%), headache after one session (0.52%), or irritation of the skin on the forehead (0.22%).
Besides official statements, there is a study focused on Cefaly users' satisfaction with the treatment.[10]
Enhancement/Therapy/Treatment
Public & Media Impact and Presentation
Cefaly has its own official facebook page since 2009, and after this, several pages about Cefaly for specific countries were created.
Public Policy
Announcement about approving migraine treat
Related Technologies, Project or Scientific Research
References
- ↑ FDA approves device to treat migraine headaches: http://articles.latimes.com/2014/mar/11/science/la-sci-sn-fda-approves-device-to-prevent-migraine-headaches-20140311
- ↑ Cefaly Technology release: http://www.biospace.com/News/cefaly-technology-release-10000-u-s-migraine/361631 (retrieved Jan 28, 2016)
- ↑ 3.0 3.1 Cefaly Set on the Cefaly official online-shop: http://www.cefalymedical.com/shop/traitement-migraine/english_a/set-cefaly.html (retrieved Jan 28, 2016)
- ↑ Cefaly® Anti-migrain Device: http://www.amazon.co.uk/Cefaly-Cefaly%C2%AE-Anti-migraine-Device/dp/B009VPAS0M (retrieved Jan 28, 2016)
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ 6.0 6.1 6.2 6.3 Two types of electrodes: http://www.cefaly.com/en/cefaly-electrodes (retrieved Jan 28, 2016)
- ↑ Occipital neuralgia on wikipedia: https://en.wikipedia.org/wiki/Occipital_neuralgia (retrieved, Jan 28, 2016)
- ↑ The Electrodes user manual: http://www.cefaly.com/en/how-to-use-it (retrieved Jan 28, 2016)
- ↑ TRYING CEFALY on Cefaly official online-shop: http://www.cefaly.com/en/cefaly-shop (retrieved Jan 28, 2016)
- ↑ 10.0 10.1 10.2 Magis D., Sava S., d’Elia T. S., Baschi R., Schoenen J., Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177534/
