Difference between revisions of "Cefaly"
From HCE Wiki - The Human Cognitive Enhancement Wiki
(→Health Risks: pic) |
(→Enhancement/Therapy/Treatment: pic) |
||
| Line 102: | Line 102: | ||
| − | [[File:Cefaly electrode effect.png|thumbnail|Electrode sends electric current through skin and affects trigeminal nerve.( | + | [[File:Cefaly electrode effect.png|thumbnail|Electrode sends electric current through skin and affects trigeminal nerve.<ref>Magis D., Sava S., d’Elia T. S., Baschi R., Schoenen J., Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177534/</ref>]] |
== Public & Media Impact and Presentation == | == Public & Media Impact and Presentation == | ||
Revision as of 22:48, 12 November 2015
| CEFALY | |
|---|---|

|
|
| Category | Therapeutic wearables |
| Developer | STX-Med |
| Announced | The date "-" was not understood. The date "-" was not understood. |
| Released | Consumers: The date "-" was not understood. The date "-" was not understood. |
| Price | 299 USD |
| Weight | g |
| Dimensions | mm |
| Controls | smartphone |
| Standalone[1] | |
| http://www.cefalytechnology.com/en/company | |
| http://www.cefalytechnology.com/en/products | |
Contents
Main characteristics
Purpose
Company & People
STX-Med company
Founders:
Pierre Rigaux
Pierre-Yves Muller
Important Dates
2004 - Foundation of STX-Med, which was later named Cefaly.[1]
Ethical Issues
Health Risks
Right on the official page of Cefaly side effects are stated.[2] Company claims, that side effects appear in 4,3% of patients, mentioning most common as intolerance to the feeling of Cefaly on the forehead (1.25%), sensation of fatigue during and after the session (0.65%), headache after one session (0.52%), or irritation of the skin on the forehead (0.22%).
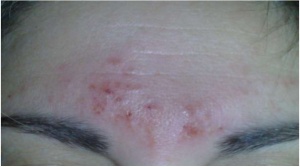
Skin irritated probably because of electrode gel containing acrylate.[2]
Enhancement/Therapy/Treatment
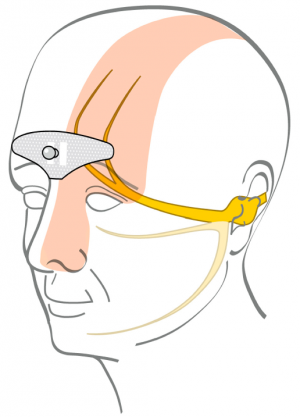
Electrode sends electric current through skin and affects trigeminal nerve.[3]
Public & Media Impact and Presentation
Cefaly has its own official facebook page since 2009, and after this, several pages about Cefaly for specific countries were created.
Public Policy
Related Technologies, Project or Scientific Research
References
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ Magis D., Sava S., d’Elia T. S., Baschi R., Schoenen J., Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177534/
- ↑ Magis D., Sava S., d’Elia T. S., Baschi R., Schoenen J., Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177534/