Difference between revisions of "Transcranial magnetic stimulation"
(added picture to historical overview) |
(added links) |
||
| Line 21: | Line 21: | ||
Late 1970s saw the creation of the [[Transcranial direct-current stimulation]] that was, together with TMS, beginning to be considered for diagnostic and therapeutic purposes among physicians. One of the first papers to utilize TSM was published in 1991.<ref>PASCUAL‐LEONE, Alvaro; GATES, John R.; DHUNA, Anil. Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology, 1991, 41.5: 697-702.</ref> The authors used repetitive TMS to induce speech difficulties in patients while counting. Other study confirmed that TMS can be successfully used in the treatment of depression in 1995.<ref>KOLBINGER, H M, HOFLICH, G, HUFNAGEL, A, MOLLER, H J and KASPER, S, 1995, Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression - a Pilot-Study. Human Psychopharmacology-Clinical and Experimental [online]. 1995. Vol. 10, no. 4, p. 305–310. DOI Doi 10.1002/Hup.470100408.</ref> The late 1990s meant wide adoption of TMS into practice, although the treatment was, and in general still is, used as a form of last resort after more established techniques fail.<ref>WASSERMANN, E M and LISANBY, S H, 2001, Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology [online]. 2001. Vol. 112, no. 8, p. 1367–77. DOI 10.1016/S1388-2457(01)00585-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11459676</ref> A consensus conference in Italy in 2008 re-evaluated the guidelines for TMS from 1998. In October 2008, the U.S. Food and Drug Administration approved the NeuroStar TMS Therapy system from Neuronetics, Inc. to be used in the industry-sponsored clinical trial of the treatment of medication-refractory depression (FDA approval K061053) through TMS.<ref>O’REARDON, John P., SOLVASON, H. Brent, JANICAK, Philip G., SAMPSON, Shirlene, ISENBERG, Keith E., NAHAS, Ziad, MCDONALD, William M., AVERY, David, FITZGERALD, Paul B., LOO, Colleen, DEMITRACK, Mark A., GEORGE, Mark S. and SACKEIM, Harold A., 2007, Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry. 2007. Vol. 62, no. 11, p. 1208–1216. DOI 10.1016/j.biopsych.2007.01.018.</ref><ref>HORVATH, Jared C, PEREZ, Jennifer M, FORROW, Lachlan, FREGNI, Felipe and PASCUAL-LEONE, Alvaro, 2011, Transcranial magnetic stimulation: a historical evaluation and future prognosis of therapeutically relevant ethical concerns. Journal of medical ethics. 2011. Vol. 37, p. 137–143. DOI 10.1136/jme.2010.039966.</ref> | Late 1970s saw the creation of the [[Transcranial direct-current stimulation]] that was, together with TMS, beginning to be considered for diagnostic and therapeutic purposes among physicians. One of the first papers to utilize TSM was published in 1991.<ref>PASCUAL‐LEONE, Alvaro; GATES, John R.; DHUNA, Anil. Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology, 1991, 41.5: 697-702.</ref> The authors used repetitive TMS to induce speech difficulties in patients while counting. Other study confirmed that TMS can be successfully used in the treatment of depression in 1995.<ref>KOLBINGER, H M, HOFLICH, G, HUFNAGEL, A, MOLLER, H J and KASPER, S, 1995, Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression - a Pilot-Study. Human Psychopharmacology-Clinical and Experimental [online]. 1995. Vol. 10, no. 4, p. 305–310. DOI Doi 10.1002/Hup.470100408.</ref> The late 1990s meant wide adoption of TMS into practice, although the treatment was, and in general still is, used as a form of last resort after more established techniques fail.<ref>WASSERMANN, E M and LISANBY, S H, 2001, Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology [online]. 2001. Vol. 112, no. 8, p. 1367–77. DOI 10.1016/S1388-2457(01)00585-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11459676</ref> A consensus conference in Italy in 2008 re-evaluated the guidelines for TMS from 1998. In October 2008, the U.S. Food and Drug Administration approved the NeuroStar TMS Therapy system from Neuronetics, Inc. to be used in the industry-sponsored clinical trial of the treatment of medication-refractory depression (FDA approval K061053) through TMS.<ref>O’REARDON, John P., SOLVASON, H. Brent, JANICAK, Philip G., SAMPSON, Shirlene, ISENBERG, Keith E., NAHAS, Ziad, MCDONALD, William M., AVERY, David, FITZGERALD, Paul B., LOO, Colleen, DEMITRACK, Mark A., GEORGE, Mark S. and SACKEIM, Harold A., 2007, Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry. 2007. Vol. 62, no. 11, p. 1208–1216. DOI 10.1016/j.biopsych.2007.01.018.</ref><ref>HORVATH, Jared C, PEREZ, Jennifer M, FORROW, Lachlan, FREGNI, Felipe and PASCUAL-LEONE, Alvaro, 2011, Transcranial magnetic stimulation: a historical evaluation and future prognosis of therapeutically relevant ethical concerns. Journal of medical ethics. 2011. Vol. 37, p. 137–143. DOI 10.1136/jme.2010.039966.</ref> | ||
| + | |||
| + | http://www.brainefit.net/history-of-tms.html | ||
| + | |||
| + | http://www.scholarpedia.org/article/Transcranial_magnetic_stimulation | ||
== Important Dates == | == Important Dates == | ||
Revision as of 11:51, 24 March 2016
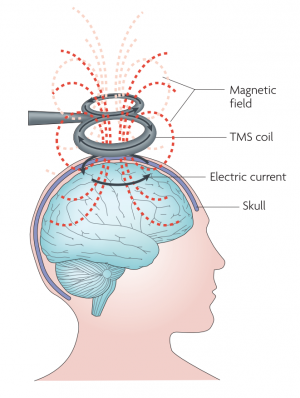
Transcranial magnetic stimulation, or TMS, is a non-invasive method of brain stimulation. During the procedure, a magnetic coil is placed over the scalp of the person receiving the treatment. The magnetic field penetrates the skull and the electric stimulus activates the neurons in the target area. This can be used to explore how the brain works, by disrupting its normal behaviour with the stimulation, or, in the case of repetitive stimulation, to modulate the brain activity for therapeutic purposes.[1][2]
Magnetic stimulation is used for mapping the human brain, mainly the primary motor cortex because the reaction of the body to the stimulus is easily observable. This is how the location of brain functions of the brain were localized, first for the motor functions, by measuring the motor evoked potentials in the muscles, and now it is possible to localize cognitive and sensory processes with TMS as well.[3]
It is also used as a treatment for major depression. Two TMS devices, NeuroStar TMS Therapy system from Neuronetics,[4] Inc., and Brainsway Deep TMS system[5] have been approved by the U.S. Food and Drug Administration for the treatment of depression[1] One session usually lasts 30 to 60 minutes.[6] for which TMS is a cost-effective alternative as opposed to antidepressants.[7][3]
Contents
Main characteristics
The magnetic field created by the the coil produces electrical current in the brain of the subject due to the electromagnetic induction effect. This produces secondary ionic current which activates the near-surface neuronal axons in the target area. However, TMS, especially in higher intensities, may activate any neuron along the way.[2] The magnetic field can reach up to 2 T and usually lasts for about 100 µm.[3]
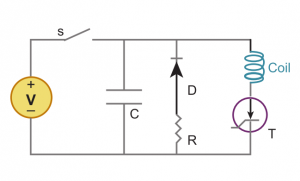
TMS system is composed of two parts. A high-voltage charge-discharge component that produces the required current waveform. This is a RLC circuit modified so it produces the waveform with the lowest amount of heat generated. The second components is the current carrying coil that servers as the emitter of the magnetic field. Although the design of the coils vary, the most used are two circular coil in parallel so as they resemble the number eight, or butterfly, shape. The coils are made of copper wiring and enclosed in a plastic chassis, with the diameter ranging from 4 to 9 centimetres.[8][9]
Purpose
Transcranial magnetic stimulation was traditionally used for brain mapping, especially of the motor cortex. However, it has been demonstrated that it can be used as a therapeutic tool also. It can remedy patients suffering from major depression and help in post-stroke rehabilitation. It can also be used to modify cognition, although the efficacy of these effects are disputed in the literature.
Historical overview
The physical principles behind magnetic field induction generating electrical current in nearby conductors were discovered by Michael Faraday in 1881. The experimentation with the effects of electricity on the human body begun in the 18th century and continued until the beginning of the 20th century. In 1937, electroconvulsive therapy was developed by the Italians Cerletti and Bini. The procedure was originally used to treat schizophrenia but became so popular it was used in virtually every psychological treatment. This resulted in a series of unwanted side-effects and the public stop tolerating the use of the technique. In 1976, the U.S. Food and Drug Administration assumed regulatory control over these types of devices.
Late 1970s saw the creation of the Transcranial direct-current stimulation that was, together with TMS, beginning to be considered for diagnostic and therapeutic purposes among physicians. One of the first papers to utilize TSM was published in 1991.[10] The authors used repetitive TMS to induce speech difficulties in patients while counting. Other study confirmed that TMS can be successfully used in the treatment of depression in 1995.[11] The late 1990s meant wide adoption of TMS into practice, although the treatment was, and in general still is, used as a form of last resort after more established techniques fail.[12] A consensus conference in Italy in 2008 re-evaluated the guidelines for TMS from 1998. In October 2008, the U.S. Food and Drug Administration approved the NeuroStar TMS Therapy system from Neuronetics, Inc. to be used in the industry-sponsored clinical trial of the treatment of medication-refractory depression (FDA approval K061053) through TMS.[13][14]
http://www.brainefit.net/history-of-tms.html
http://www.scholarpedia.org/article/Transcranial_magnetic_stimulation
Important Dates
Enhancement/Therapy/Treatment
Ethical & Health Issues
Public & Media Impact and Presentation
Public Policy
Related Technologies, Projects or Scientific Research
References
- ↑ 1.0 1.1 ELDAIEF, Mark C., PRESS, Daniel Z. and PASCUAL-LEONE, Alvaro, 2013, Transcranial magnetic stimulation in neurology A review of established and prospective applications. Neurology: Clinical Practice. 2013. Vol. 3, no. 6, p. 519–526. DOI 10.1212/01.CPJ.0000436213.11132.8e.
- ↑ 2.0 2.1 RIDDING, Michael C and ROTHWELL, John C, 2007, Is there a future for therapeutic use of transcranial magnetic stimulation? Nature reviews. Neuroscience. 2007. Vol. 8, no. 7, p. 559–567. DOI 10.1038/nrn2169.
- ↑ 3.0 3.1 3.2 HALLETT, Mark, 2007, Transcranial Magnetic Stimulation: A Primer. Neuron. 2007. Vol. 55, no. 2, p. 187–199. DOI 10.1016/j.neuron.2007.06.026.
- ↑ https://neurostar.com/
- ↑ http://www.brainsway.com/about-brainsway
- ↑ http://www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml
- ↑ NGUYEN, Kim-Huong and GORDON, Louisa G, 2015, Cost-Effectiveness of Repetitive Transcranial Magnetic Stimulation versus Antidepressant Therapy for Treatment-Resistant Depression. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research [online]. 2015. Vol. 18, no. 5, p. 597–604. DOI 10.1016/j.jval.2015.04.004. Available from: http://www.sciencedirect.com/science/article/pii/S1098301515019270
- ↑ WAGNER, Timothy, VALERO-CABRE, Antoni and PASCUAL-LEONE, Alvaro, 2007, Noninvasive human brain stimulation. Annual Review of Biomedical Engineering [online]. 2007. Vol. 9, no. 1, p. 527–565. DOI 10.1146/annurev.bioeng.9.061206.133100. Available from: http://dx.doi.org/10.1146/annurev.bioeng.9.061206.133100\nfiles/392/Wagner et al. - 2007 - Noninvasive Human Brain Stimulation.pdf
- ↑ KOBAYASHI, Masahito and PASCUAL-LEONE, Alvaro, 2003, Basic principles of magnetic stimulation. The Lancet. 2003. Vol. 2, p. 145–156. DOI 10.1016/S1474-4422(03)00321-1.
- ↑ PASCUAL‐LEONE, Alvaro; GATES, John R.; DHUNA, Anil. Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology, 1991, 41.5: 697-702.
- ↑ KOLBINGER, H M, HOFLICH, G, HUFNAGEL, A, MOLLER, H J and KASPER, S, 1995, Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression - a Pilot-Study. Human Psychopharmacology-Clinical and Experimental [online]. 1995. Vol. 10, no. 4, p. 305–310. DOI Doi 10.1002/Hup.470100408.
- ↑ WASSERMANN, E M and LISANBY, S H, 2001, Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology [online]. 2001. Vol. 112, no. 8, p. 1367–77. DOI 10.1016/S1388-2457(01)00585-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11459676
- ↑ O’REARDON, John P., SOLVASON, H. Brent, JANICAK, Philip G., SAMPSON, Shirlene, ISENBERG, Keith E., NAHAS, Ziad, MCDONALD, William M., AVERY, David, FITZGERALD, Paul B., LOO, Colleen, DEMITRACK, Mark A., GEORGE, Mark S. and SACKEIM, Harold A., 2007, Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry. 2007. Vol. 62, no. 11, p. 1208–1216. DOI 10.1016/j.biopsych.2007.01.018.
- ↑ HORVATH, Jared C, PEREZ, Jennifer M, FORROW, Lachlan, FREGNI, Felipe and PASCUAL-LEONE, Alvaro, 2011, Transcranial magnetic stimulation: a historical evaluation and future prognosis of therapeutically relevant ethical concerns. Journal of medical ethics. 2011. Vol. 37, p. 137–143. DOI 10.1136/jme.2010.039966.